Qsymia is a FDA (U.S. Food and Medication Organization) supported medicine fabricated by Vivus, Inc. It is utilized with a diminished calorie diet and an expansion in actual work for ongoing weight the executives in grown-ups with a weight file (BMI) of 30 kg/m2 or more (hefty), or 27 kg/m2 or more (overweight) with something like one weight-related comorbidity like hypertension (hypertension), type 2 diabetes mellitus, or dyslipidemia (elevated cholesterol). While Qsymia is by and large all around endured, there are a few potential secondary effects that individuals ought to know about. The most well-known results of Qsymia incorporate trouble dozing. More uncommon, yet more serious incidental effects, can incorporate self-destructive contemplations or conduct.
Get familiar with the symptoms of Qsymia and how you might stay away from them.
What is Qsymia (phentermine and topiramate)?
Qsymia contains phentermine, a hunger suppressant having a place with a class of medications called sympathomimetic amines, and topiramate, an anticonvulsant. As Qsymia contains phentermine, a Timetable IV medication, it also is controlled under Timetable IV of the Controlled Substances Act. Qsymia is just accessible under an exceptional program called the Qsymia REMS program.
Qsymia dose
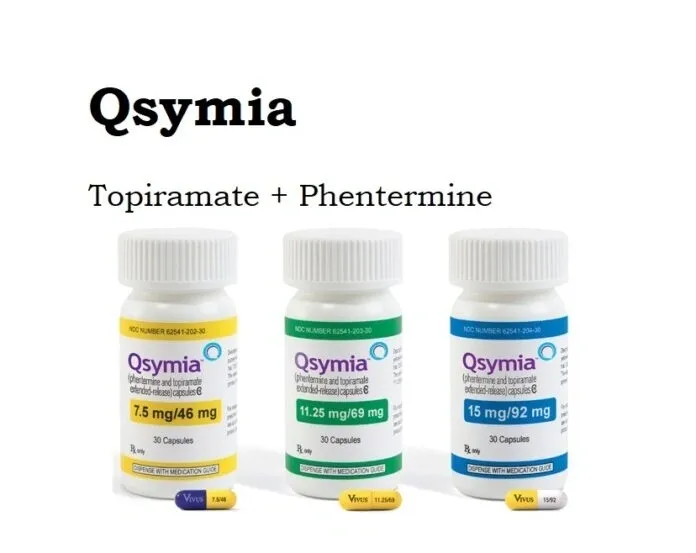
Qsymia is accessible in container structure, in the accompanying dosages: phentermine/topiramate broadened discharge 3.75 mg/23 mg, 7.5 mg/46 mg, 11.25 mg/69 mg, or 15 mg/92 mg.
The suggested portion of Qsymia is 3.75 mg/23 mg day to day for 14 days, then expanded to 7.5 mg/46 mg day to day. Weight reduction ought to be really looked at following 12 weeks. On the off chance that a patient has not lost at least 3% of pattern body weight, stop treatment or diminish the portion as it is impossible the patient will accomplish and keep up with clinically significant weight reduction at this portion. In the event that you miss a portion of Qsymia, skirt the missed portion and take your next portion at the ordinary time. Try not to twofold your portion to get up to speed.
You are encouraged to peruse the prescription aide gave this medication for the medication data and patient data, and consistently talk with your medical care supplier for clinical counsel about any progressions to your portion so they can screen and assess your condition.
Qsymia secondary effects
The most widely recognized symptoms of Qsymia capsules in clinical preliminaries contrasted with fake treatment include:
Paraesthesia
Skin responses
Back torment
Tipsiness
Dysgeusia
Inconvenience resting
Sluggishness
Stoppage
Dry mouth
In uncommon examples, Qsymia capsules can cause more serious secondary effects. These can include:
Fetal Poisonousness – females of regenerative potential ought to get a negative pregnancy test before treatment and month to month from that point onward, and utilize viable contraception.
Your primary care physician will evaluate the advantages of utilizing Qsymia against your gamble of aftereffects. Patients are urged to report negative aftereffects or unfriendly responses of Qsymia to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
What are the drawn out results of Qsymia?
Qsymia capsules might cause serious aspiratory hypertension, and heart valve issues. The gamble of this happening increments with long haul use and whenever utilized with other craving suppressants.
Does Qsymia cause balding?
Topiramate, one of the dynamic fixings in Qsymia has been believed to cause going bald. This is definitely not a typical secondary effect and has just been accounted for in 1% to 4% of kids.
Expansion in pulse
Self-destructive contemplations or ideation
Intense nearsightedness and auxiliary point conclusion glaucoma
Mind-set changes and rest issues
Mental weakness
Metabolic acidosis – loss of hunger, sleepiness, thinking issues, unpredictable pulses
Raised creatinine
Diminished perspiring and expanded internal heat level
Unexpected changes in vision, for example, diminished vision, obscured vision, eye torment/redness
Your primary care physician will evaluate the advantages of utilizing Qsymia against your gamble of aftereffects. Patients are urged to report negative aftereffects or unfriendly responses of Qsymia to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
What are the drawn out results of Qsymia?
Qsymia capsules might cause serious aspiratory hypertension, and heart valve issues. The gamble of this happening increments with long haul use and whenever utilized with other craving suppressants.
Does Qsymia cause balding?
Topiramate, one of the dynamic fixings in Qsymia has been believed to cause going bald. This is definitely not a typical secondary effect and has just been accounted for in 1% to 4% of kids.